| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-23 21:03:03 UTC |
|---|
| Update Date | 2014-12-24 20:24:47 UTC |
|---|
| Accession Number | T3D1866 |
|---|
| Identification |
|---|
| Common Name | Diantimony diselenide |
|---|
| Class | Small Molecule |
|---|
| Description | Diantimony diselenide is a chemical compound of antimony and selenium. Antimony is a metallic element with the chemical symbol Sb and atomic number 51. Small amounts of antimony are found in the earth's crust. (8, 9) |
|---|
| Compound Type | - Antimony Compound
- Food Toxin
- Inorganic Compound
- Pollutant
- Selenium Compound
- Synthetic Compound
|
|---|
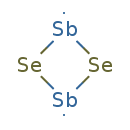
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Antimony selenide | | Sb2Se2 |
|
|---|
| Chemical Formula | Sb2Se2 |
|---|
| Average Molecular Mass | 401.440 g/mol |
|---|
| Monoisotopic Mass | 401.641 g/mol |
|---|
| CAS Registry Number | 193095-46-4 |
|---|
| IUPAC Name | 1,3,2,4-diselenadistibetan-2-yl |
|---|
| Traditional Name | 1,3,2,4-diselenadistibetan-2-yl |
|---|
| SMILES | [Se]1[Sb][Se][Sb]1 |
|---|
| InChI Identifier | InChI=1S/2Sb.2Se |
|---|
| InChI Key | InChIKey=FYJNARDRSABDPB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as inorganic antimony salts. These are inorganic salts of antimony. They usually contain antimony in its ionic form. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Inorganic salts |
|---|
| Class | Inorganic antimony salts |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Inorganic antimony salts |
|---|
| Alternative Parents | |
|---|
| Substituents | - Inorganic antimony salt
- Metalloheterocycle
- Inorganic selenide
- Miscellaneous mixed metal/non-metal
- Inorganic metalloid salt
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | Not Available |
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation (8) ; oral (8) ; dermal (8) |
|---|
| Mechanism of Toxicity | The inhalation data suggests that the myocardium is a target of antimony toxicity. It is possible that antimony affects circulating glucose by interfering with enzymes of the glycogenolysis and gluconeogenesis pathways. The mechanism of action of antimony remains unclear. However, some studies suggest that antimony combines with sulfhydryl groups including those in several enzymes important for tissue respiration. The antidotal action of BAL depends on its ability to prevent or break the union between antimony and vital enzymes. Moreover, the The cause of death is believed to be essentially the same as that in acute arsenic poisoning. Selenium readily substitutes for sulfur in biomolecules and in many biochemical reactions, especially when the concentration of selenium is high and the concentration of sulfur is low. Inactivation of the sulfhydryl enzymes necessary for oxidative reactions in cellular respiration, through effects on mitochondrial and microsomal electron transport, might contribute to acute selenium toxicity. Selenomethionine (a common organic selenium compound) also appears to randomly substitute for methionine in protein synthesis. This substitution may affect the structure and functionability of the protein, for example, by altering disulfide bridges. Inorganic forms of selenium appear to react with tissue thiols by redox catalysis, resulting in formation of reactive oxygen species and causing damage by oxidative stress. (2, 7, 8, 1). |
|---|
| Metabolism | Antimony is widely distributed throughout the body. The hair and skin contain the highest levels of the compound. The adrenal glands, lung, large intestine, trachea, cerebellum, and kidneys also contain relatively high levels of it. Blood is the main vehicle for the transport of absorbed antimony to various tissue compartments of the body. The exact metabolic pathway of antimony remains unclear. Antimony is excreted via the urine and feces. Some of the fecal antimony may represent unabsorbed antimony that is cleared from the lung via mucociliary action into the esophagus to the gastrointestinal tract. Selenium may be absorbed through inhalation and ingestion, while some selenium compounds may also be absorbed dermally. Once in the body, selenium is distributed mainly to the liver and kidney. Selenium is an essential micronutrient and is a component of glutathione peroxidase, iodothyronine 5'-deiodinases, and thioredoxin reductase. Organic selenium is first metabolized into inorganic selenium. Inorganic selenium is reduced stepwise to the intermediate hydrogen selenide, which is either incorporated into selenoproteins after being transformed to selenophosphate and selenocysteinyl tRNA or excreted into the urine after being transformed into methylated metabolites of selenide. Elemental selenium is also methylated before excretion. Selenium is primarily eliminated in the urine and feces, but certain selenium compounds may also be exhaled. (7, 8). |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (6) |
|---|
| Uses/Sources | Breathing air, drinking water, and eating foods that contain antimony. Exposure can also occur through dermal or skin contact (8). |
|---|
| Minimum Risk Level | Chronic Oral: 0.005 mg/kg/day (Selenium) (5) |
|---|
| Health Effects | Antimony poisoning can lead to pneumoconiosis. Alterations in pulmonary function and other effetcs including chronic bronchitis, chronic emphysema, inactive tuberculosis, pleural adhesions, and irritation can result from inhalation of antimony. Chronic oral exposure to high concentrations of selenium compounds can produce a disease called selenosis. The major signs of selenosis are hair loss, nail brittleness, and neurological abnormalities (such as numbness and other odd sensations in the extremities). Animal studies have shown that selenium may also affect sperm production and the female reproductive cycle. (7, 8) |
|---|
| Symptoms | Abdominal pain, vomiting, diarrhea, and loss of appetite can result from ingestion of antimony. Dyspnea, headache, vomiting,cough, conjunctivitis, and bloody purulent discharge from nose can result from inhalation exposure. Skin or eye contact can cause pain and redness of the exposed surface. Severe burns of the cornea can result from eye exposure. Short-term oral exposure to high concentrations of selenium may cause nausea, vomiting, and diarrhea. Brief exposures to high levels of elemental selenium or selenium dioxide in air can result in respiratory tract irritation, bronchitis, difficulty breathing, and stomach pains. Longer-term exposure to either of these air-borne forms can cause respiratory irritation, bronchial spasms, and coughing. (7, 4, 10) |
|---|
| Treatment | Following oral exposure to antimony, administer charcoal as a slurry (240 mL water/30 g charcoal). Following inhalation exposure, move patient to fresh air. Monitor for respiratory distress. If cough or difficulty breathing develops, evaluate for respiratory tract irritation, bronchitis, or pneumonitis. Administer oxygen and assist ventilation as required. Treat bronchospasm with inhaled beta2 agonist and oral or parenteral corticosteroids. In case of eye exposure, irrigate exposed eyes with copious amounts of room temperature water for at least 15 minutes. Following dermal exposure, Remove contaminated clothing and wash exposed area thoroughly with soap and water. A physician may need to examine the area if irritation or pain persists. (3) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 6336250 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Diantimony diselenide |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Poon R, Chu I, Lecavalier P, Valli VE, Foster W, Gupta S, Thomas B: Effects of antimony on rats following 90-day exposure via drinking water. Food Chem Toxicol. 1998 Jan;36(1):21-35. [9487361 ]
- Hayes WJ Jr. and Laws ER Jr. (eds) (1991). Handbook of Pesticide Toxicology. Volume 3. Classes of Pesticides. New York, NY: Academic Press, Inc.
- Rumack BH (2009). POISINDEX(R) Information System. Englewood, CO: Micromedex, Inc. CCIS Volume 141, edition expires Aug, 2009.
- Hamilton A and Hardy HL (1974). Industrial Toxicology. 3rd ed. Acton, MA: Publishing Sciences Group, Inc.
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for selenium. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1992). Toxicological profile for antimony. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1990). Toxicological profile for silver. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2005). Toxicological profile for zinc. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|