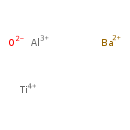
Aluminium barium titanium oxide (T3D1091)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-06-19 21:58:17 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:23:07 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D1091 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Aluminium barium titanium oxide | ||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Aluminium barium titanium oxide is an oxide of aluminum, barium, and titanium. Moderately toxic by intraperitoneal route. Low toxicity by ingestion. When heated to decomposition it emits toxic vapors of Ba and Ti. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | AlBaOTi | ||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 228.171 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 228.826 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 52869-91-7 | ||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | titanium(4+) ion aluminium(3+) ion barium(2+) ion oxidandiide | ||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | titanium(4+) ion aluminium(3+) ion barium(2+) ion oxidandiide | ||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [O--].[Al+3].[Ti+4].[Ba++] | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/Al.Ba.O.Ti/q+3;+2;-2;+4 | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=LTRINLFGMOIOAG-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of inorganic compounds known as post-transition metal oxides. These are inorganic compounds containing an oxygen atom of an oxidation state of -2, in which the heaviest atom bonded to the oxygen is a post-transition metal. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Mixed metal/non-metal compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Post-transition metal organides | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Post-transition metal oxides | ||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Post-transition metal oxides | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Oral (6) ; inhalation (6) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Barium is a competitive potassium channel antagonist that blocks the passive efflux of intracellular potassium, resulting in a shift of potassium from extracellular to intracellular compartments. The intracellular translocation of potassium results in a decreased resting membrane potential, making the muscle fibers electrically unexcitable and causing paralysis. Some of these barium's effects may also be due to barium induced neuromuscular blockade and membrane depolarization. The main target organs of aluminum are the central nervous system and bone. Aluminum binds with dietary phosphorus and impairs gastrointestinal absorption of phosphorus. The decreased phosphate body burden results in osteomalacia (softening of the bones due to defective bone mineralization) and rickets. Aluminum's neurotoxicity is believed to involve several mechanisms. Changes in cytoskeletal protein functions as a results of altered phosphorylation, proteolysis, transport, and synthesis are believed to be one cause. Aluminum may induce neurobehavioral effects by affecting permeability of the blood-brain barrier, cholinergic activity, signal transduction pathways, lipid peroxidation, and impair neuronal glutamate nitric oxide-cyclic GMP pathway, as well as interfere with metabolism of essential trace elements because of similar coordination chemistries and consequent competitive interactions. It has been suggested that aluminum's interaction with estrogen receptors increases the expression of estrogen-related genes and thereby contributes to the progression of breast cancer (1), but studies have not been able to establish a clear link between aluminum and increased risk of breast cancer (3). Certain aluminum salts induce immune responses by activating inflammasomes. (7, 1, 2, 6) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Barium compounds are absorbed via ingestion and inhalation, the extent of which depends on the individual compound. In the body, the majority of the barium is found in the bone, while small amounts exists in the muscle, adipose, skin, and connective tissue. Barium is not metabolized in the body, but it may be transported or incorporated into complexes or tissues. Barium is excreted in the urine and faeces. Aluminum is poorly absorbed following either oral or inhalation exposure and is essentially not absorbed dermally. The bioavailability of aluminum is strongly influenced by the aluminum compound and the presence of dietary constituents which can complex with aluminum and enhance or inhibit its absorption. Aluminum binds to various ligands in the blood and distributes to every organ, with highest concentrations found in bone and lung tissues. In living organisms, aluminum is believed to exist in four different forms: as free ions, as low-molecular-weight complexes, as physically bound macromolecular complexes, and as covalently bound macromolecular complexes. Absorbed aluminum is excreted principally in the urine and, to a lesser extent, in the bile, while unabsorbed aluminum is excreted in the faeces. (7, 6) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | 1 to 15 grams for an adult human (barium salts). (4) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | Not listed by IARC. IARC classified aluminum production as carcinogenic to humans (Group 1), but did not implicate aluminum itself as a human carcinogen. (9) A link between use of aluminum-containing antiperspirants and increased risk of breast cancer has been proposed (1), but studies have not been able to establish a clear link (3). | ||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Intermediate Oral: 0.2 mg/kg/day (Barium) (5) Chronic Oral: 0.2 mg/kg/day (Barium) (5) Intermediate Oral: 1.0 mg/kg/day (Aluminum) (5) Chronic Oral: 1.0 mg/kg/day (Aluminum) (5) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | The health effects of the different barium compounds depend on how well the compound dissolves in water or the stomach contents. At low doses, barium acts as a muscle stimulant, while higher doses affect the nervous system, causing cardiac irregularities, tremors, weakness, anxiety, dyspnea, paralysisand possibly death. Barium may also cause gastrointestinal disturbances, damage the kidneys and cause decreases in body weight. Aluminum targets the nervous system and causes decreased nervous system performance and is associated with altered function of the blood-brain barrier. The accumulation of aluminum in the body may cause bone or brain diseases. High levels of aluminum have been linked to Alzheimer’s disease. A small percentage of people are allergic to aluminium and experience contact dermatitis, digestive disorders, vomiting or other symptoms upon contact or ingestion of products containing aluminium. (7, 8, 6) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Ingesting excess barium may cause vomiting, abdominal cramps, diarrhea, difficulties in breathing, increased or decreased blood pressure, numbness around the face, and muscle weakness. High levels may result in changes in heart rhythm or paralysis and possibly death. Inhalating aluminum dust causes coughing and abnormal chest X-rays. A small percentage of people are allergic to aluminium and experience contact dermatitis, digestive disorders, vomiting or other symptoms upon contact or ingestion of products containing aluminium. (7, 8, 6) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Intravenous infusion of potassium often relieves many of the symptoms of barium toxicity. (6) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 171218 | ||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 149685 | ||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Aluminium barium titanium oxide | ||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Phosphatidylinositol-4,5-bisphosphate binding
- Specific Function:
- In the kidney, probably plays a major role in potassium homeostasis. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. This channel is activated by internal ATP and can be blocked by external barium.
- Gene Name:
- KCNJ1
- Uniprot ID:
- P48048
- Molecular Weight:
- 44794.6 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- This receptor is controlled by G proteins. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. Can be blocked by extracellular barium (By similarity). Subunit of ATP-sensitive potassium channels (KATP). Can form cardiac and smooth muscle-type KATP channels with ABCC9. KCNJ11 forms the channel pore while ABCC9 is required for activation and regulation.
- Gene Name:
- KCNJ11
- Uniprot ID:
- Q14654
- Molecular Weight:
- 43540.375 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Inward rectifier potassium channel activity
- Specific Function:
- Inward rectifying potassium channel that is activated by phosphatidylinositol 4,5-bisphosphate and that probably participates in controlling the resting membrane potential in electrically excitable cells. Probably participates in establishing action potential waveform and excitability of neuronal and muscle tissues. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium.
- Gene Name:
- KCNJ12
- Uniprot ID:
- Q14500
- Molecular Weight:
- 49000.6 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Inward rectifier potassium channel activity
- Specific Function:
- This potassium channel is controlled by G proteins. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. Can be blocked by external barium (By similarity).
- Gene Name:
- KCNJ8
- Uniprot ID:
- Q15842
- Molecular Weight:
- 47967.455 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
5. ATP-sensitive potassium channel (Protein Group)
- General Function:
- Phosphatidylinositol-4,5-bisphosphate binding
- Specific Function:
- In the kidney, probably plays a major role in potassium homeostasis. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. This channel is activated by internal ATP and can be blocked by external barium.
- Included Proteins:
- P48048 , P78508 , Q14654 , Q14500 , Q9UNX9 , Q99712 , Q15842
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
6. ATP-sensitive potassium channel (Protein Group)
- General Function:
- Phosphatidylinositol-4,5-bisphosphate binding
- Specific Function:
- In the kidney, probably plays a major role in potassium homeostasis. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. This channel is activated by internal ATP and can be blocked by external barium.
- Included Proteins:
- P48048 , P78508 , Q14654 , Q14500 , Q9UNX9 , Q99712 , Q15842
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
7. ATP-sensitive potassium channel (Protein Group)
- General Function:
- Phosphatidylinositol-4,5-bisphosphate binding
- Specific Function:
- In the kidney, probably plays a major role in potassium homeostasis. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. This channel is activated by internal ATP and can be blocked by external barium.
- Included Proteins:
- P48048 , P78508 , Q14654 , Q14500 , Q9UNX9 , Q99712 , Q15842
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Titin binding
- Specific Function:
- Calmodulin mediates the control of a large number of enzymes, ion channels, aquaporins and other proteins by Ca(2+). Among the enzymes to be stimulated by the calmodulin-Ca(2+) complex are a number of protein kinases and phosphatases. Together with CCP110 and centrin, is involved in a genetic pathway that regulates the centrosome cycle and progression through cytokinesis.
- Gene Name:
- CALM1
- Uniprot ID:
- P0DP23
- Molecular Weight:
- 16837.47 Da
References
- Kursula P, Majava V: A structural insight into lead neurotoxicity and calmodulin activation by heavy metals. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007 Aug 1;63(Pt 8):653-6. Epub 2007 Jul 28. [17671360 ]
- General Function:
- Zinc ion binding
- Specific Function:
- Nuclear hormone receptor. The steroid hormones and their receptors are involved in the regulation of eukaryotic gene expression and affect cellular proliferation and differentiation in target tissues. Ligand-dependent nuclear transactivation involves either direct homodimer binding to a palindromic estrogen response element (ERE) sequence or association with other DNA-binding transcription factors, such as AP-1/c-Jun, c-Fos, ATF-2, Sp1 and Sp3, to mediate ERE-independent signaling. Ligand binding induces a conformational change allowing subsequent or combinatorial association with multiprotein coactivator complexes through LXXLL motifs of their respective components. Mutual transrepression occurs between the estrogen receptor (ER) and NF-kappa-B in a cell-type specific manner. Decreases NF-kappa-B DNA-binding activity and inhibits NF-kappa-B-mediated transcription from the IL6 promoter and displace RELA/p65 and associated coregulators from the promoter. Recruited to the NF-kappa-B response element of the CCL2 and IL8 promoters and can displace CREBBP. Present with NF-kappa-B components RELA/p65 and NFKB1/p50 on ERE sequences. Can also act synergistically with NF-kappa-B to activate transcription involving respective recruitment adjacent response elements; the function involves CREBBP. Can activate the transcriptional activity of TFF1. Also mediates membrane-initiated estrogen signaling involving various kinase cascades. Isoform 3 is involved in activation of NOS3 and endothelial nitric oxide production. Isoforms lacking one or several functional domains are thought to modulate transcriptional activity by competitive ligand or DNA binding and/or heterodimerization with the full length receptor. Essential for MTA1-mediated transcriptional regulation of BRCA1 and BCAS3. Isoform 3 can bind to ERE and inhibit isoform 1.
- Gene Name:
- ESR1
- Uniprot ID:
- P03372
- Molecular Weight:
- 66215.45 Da
References
- Darbre PD: Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J Appl Toxicol. 2006 May-Jun;26(3):191-7. [16489580 ]
- General Function:
- G-protein activated inward rectifier potassium channel activity
- Specific Function:
- This potassium channel is controlled by G proteins. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. This receptor plays a crucial role in regulating the heartbeat.
- Gene Name:
- KCNJ3
- Uniprot ID:
- P48549
- Molecular Weight:
- 56602.84 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Inward rectifier potassium channel activity
- Specific Function:
- This potassium channel may be involved in the regulation of insulin secretion by glucose and/or neurotransmitters acting through G-protein-coupled receptors. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium.
- Gene Name:
- KCNJ6
- Uniprot ID:
- P48051
- Molecular Weight:
- 48450.96 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- G-protein activated inward rectifier potassium channel activity
- Specific Function:
- This receptor is controlled by G proteins. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium (By similarity).
- Gene Name:
- KCNJ9
- Uniprot ID:
- Q92806
- Molecular Weight:
- 44019.45 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- G-protein activated inward rectifier potassium channel activity
- Specific Function:
- This potassium channel is controlled by G proteins. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. Can be blocked by external barium.
- Gene Name:
- KCNJ5
- Uniprot ID:
- P48544
- Molecular Weight:
- 47667.3 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Inward rectifier potassium channel activity
- Specific Function:
- Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. KCNJ13 has a very low single channel conductance, low sensitivity to block by external barium and cesium, and no dependence of its inward rectification properties on the internal blocking particle magnesium.
- Gene Name:
- KCNJ13
- Uniprot ID:
- O60928
- Molecular Weight:
- 40529.195 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Inward rectifier potassium channel activity
- Specific Function:
- Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. KCNJ16 may be involved in the regulation of fluid and pH balance.
- Gene Name:
- KCNJ16
- Uniprot ID:
- Q9NPI9
- Molecular Weight:
- 47948.585 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Voltage-gated potassium channel activity involved in cardiac muscle cell action potential repolarization
- Specific Function:
- Probably participates in establishing action potential waveform and excitability of neuronal and muscle tissues. Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. Can be blocked by extracellular barium or cesium.
- Gene Name:
- KCNJ2
- Uniprot ID:
- P63252
- Molecular Weight:
- 48287.82 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Pdz domain binding
- Specific Function:
- Inward rectifier potassium channels are characterized by a greater tendency to allow potassium to flow into the cell rather than out of it. Their voltage dependence is regulated by the concentration of extracellular potassium; as external potassium is raised, the voltage range of the channel opening shifts to more positive voltages. The inward rectification is mainly due to the blockage of outward current by internal magnesium. Can be blocked by extracellular barium and cesium (By similarity).
- Gene Name:
- KCNJ4
- Uniprot ID:
- P48050
- Molecular Weight:
- 49499.61 Da
References
- Alagem N, Dvir M, Reuveny E: Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001 Jul 15;534(Pt. 2):381-93. [11454958 ]
- General Function:
- Transcription factor binding
- Specific Function:
- As the sensor component of the NLRP3 inflammasome, plays a crucial role in innate immunity and inflammation. In response to pathogens and other damage-associated signals, initiates the formation of the inflammasome polymeric complex, made of NLRP3, PYCARD and CASP1 (and possibly CASP4 and CASP5). Recruitement of proCASP1 to the inflammasome promotes its activation and CASP1-catalyzed IL1B and IL18 maturation and secretion in the extracellular milieu. Activation of NLRP3 inflammasome is also required for HMGB1 secretion (PubMed:22801494). The active cytokines and HMGB1 stimulate inflammatory responses. Inflammasomes can also induce pyroptosis, an inflammatory form of programmed cell death. Under resting conditions, NLRP3 is autoinhibited. NLRP3 activation stimuli include extracellular ATP, reactive oxygen species, K(+) efflux, crystals of monosodium urate or cholesterol, beta-amyloid fibers, environmental or industrial particles and nanoparticles, etc. However, it is unclear what constitutes the direct NLRP3 activator. Independently of inflammasome activation, regulates the differentiation of T helper 2 (Th2) cells and has a role in Th2 cell-dependent asthma and tumor growth (By similarity). During Th2 differentiation, required for optimal IRF4 binding to IL4 promoter and for IRF4-dependent IL4 transcription. Binds to the consensus DNA sequence 5'-GRRGGNRGAG-3'. May also participate in the transcription of IL5, IL13, GATA3, CCR3, CCR4 and MAF (By similarity).
- Gene Name:
- NLRP3
- Uniprot ID:
- Q96P20
- Molecular Weight:
- 118171.375 Da
References
- Aimanianda V, Haensler J, Lacroix-Desmazes S, Kaveri SV, Bayry J: Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol Sci. 2009 Jun;30(6):287-95. doi: 10.1016/j.tips.2009.03.005. Epub 2009 May 11. [19439372 ]
References
- ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for aluminum. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- General Function:
- Voltage-gated potassium channel activity involved in ventricular cardiac muscle cell action potential repolarization
- Specific Function:
- Potassium channel that plays an important role in a number of tissues, including heart, inner ear, stomach and colon (By similarity) (PubMed:10646604). Associates with KCNE beta subunits that modulates current kinetics (By similarity) (PubMed:9312006, PubMed:9108097, PubMed:8900283, PubMed:10646604, PubMed:11101505, PubMed:19687231). Induces a voltage-dependent by rapidly activating and slowly deactivating potassium-selective outward current (By similarity) (PubMed:9312006, PubMed:9108097, PubMed:8900283, PubMed:10646604, PubMed:11101505). Promotes also a delayed voltage activated potassium current showing outward rectification characteristic (By similarity). During beta-adrenergic receptor stimulation participates in cardiac repolarization by associating with KCNE1 to form the I(Ks) cardiac potassium current that increases the amplitude and slows down the activation kinetics of outward potassium current I(Ks) (By similarity) (PubMed:9312006, PubMed:9108097, PubMed:8900283, PubMed:10646604, PubMed:11101505). Muscarinic agonist oxotremorine-M strongly suppresses KCNQ1/KCNE1 current (PubMed:10713961). When associated with KCNE3, forms the potassium channel that is important for cyclic AMP-stimulated intestinal secretion of chloride ions (PubMed:10646604). This interaction with KCNE3 is reduced by 17beta-estradiol, resulting in the reduction of currents (By similarity). During conditions of increased substrate load, maintains the driving force for proximal tubular and intestinal sodium ions absorption, gastric acid secretion, and cAMP-induced jejunal chloride ions secretion (By similarity). Allows the provision of potassium ions to the luminal membrane of the secretory canaliculus in the resting state as well as during stimulated acid secretion (By similarity). When associated with KCNE2, forms an heterooligomer complex leading to currents with an apparently instantaneous activation, a rapid deactivation process and a linear current-voltage relationship and decreases the amplitude of the outward current (PubMed:11101505). When associated with KCNE4, inhibits voltage-gated potassium channel activity (PubMed:19687231). When associated with KCNE5, this complex only conducts current upon strong and continued depolarization (PubMed:12324418). Also forms an heterotetramer with KCNQ5; has a voltage-gated potassium channel activity (PubMed:24855057). Binds with phosphatidylinositol 4,5-bisphosphate (PubMed:25037568).Isoform 2: Non-functional alone but modulatory when coexpressed with the full-length isoform 1.
- Gene Name:
- KCNQ1
- Uniprot ID:
- P51787
- Molecular Weight:
- 74697.925 Da
References
- Gibor G, Yakubovich D, Peretz A, Attali B: External barium affects the gating of KCNQ1 potassium channels and produces a pore block via two discrete sites. J Gen Physiol. 2004 Jul;124(1):83-102. [15226366 ]
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- Probably important in the regulation of neuronal excitability. Associates with KCNQ3 to form a potassium channel with essentially identical properties to the channel underlying the native M-current, a slowly activating and deactivating potassium conductance which plays a critical role in determining the subthreshold electrical excitability of neurons as well as the responsiveness to synaptic inputs. KCNQ2/KCNQ3 current is blocked by linopirdine and XE991, and activated by the anticonvulsant retigabine. Muscarinic agonist oxotremorine-M strongly suppress KCNQ2/KCNQ3 current in cells in which cloned KCNQ2/KCNQ3 channels were coexpressed with M1 muscarinic receptors.
- Gene Name:
- KCNQ2
- Uniprot ID:
- O43526
- Molecular Weight:
- 95846.575 Da
References
- Gibor G, Yakubovich D, Peretz A, Attali B: External barium affects the gating of KCNQ1 potassium channels and produces a pore block via two discrete sites. J Gen Physiol. 2004 Jul;124(1):83-102. [15226366 ]
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- Probably important in the regulation of neuronal excitability. Associates with KCNQ2 or KCNQ5 to form a potassium channel with essentially identical properties to the channel underlying the native M-current, a slowly activating and deactivating potassium conductance which plays a critical role in determining the subthreshold electrical excitability of neurons as well as the responsiveness to synaptic inputs.
- Gene Name:
- KCNQ3
- Uniprot ID:
- O43525
- Molecular Weight:
- 96741.515 Da
References
- Gibor G, Yakubovich D, Peretz A, Attali B: External barium affects the gating of KCNQ1 potassium channels and produces a pore block via two discrete sites. J Gen Physiol. 2004 Jul;124(1):83-102. [15226366 ]
- General Function:
- Potassium channel activity
- Specific Function:
- Probably important in the regulation of neuronal excitability. May underlie a potassium current involved in regulating the excitability of sensory cells of the cochlea. KCNQ4 channels are blocked by linopirdin, XE991 and bepridil, whereas clofilium is without significant effect. Muscarinic agonist oxotremorine-M strongly suppress KCNQ4 current in CHO cells in which cloned KCNQ4 channels were coexpressed with M1 muscarinic receptors.
- Gene Name:
- KCNQ4
- Uniprot ID:
- P56696
- Molecular Weight:
- 77099.99 Da
References
- Gibor G, Yakubovich D, Peretz A, Attali B: External barium affects the gating of KCNQ1 potassium channels and produces a pore block via two discrete sites. J Gen Physiol. 2004 Jul;124(1):83-102. [15226366 ]
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- Probably important in the regulation of neuronal excitability. Associates with KCNQ3 to form a potassium channel which contributes to M-type current, a slowly activating and deactivating potassium conductance which plays a critical role in determining the subthreshold electrical excitability of neurons. May contribute, with other potassium channels, to the molecular diversity of a heterogeneous population of M-channels, varying in kinetic and pharmacological properties, which underlie this physiologically important current. Insensitive to tetraethylammonium, but inhibited by barium, linopirdine and XE991. Activated by niflumic acid and the anticonvulsant retigabine. Muscarine suppresses KCNQ5 current in Xenopus oocytes in which cloned KCNQ5 channels were coexpressed with M(1) muscarinic receptors.
- Gene Name:
- KCNQ5
- Uniprot ID:
- Q9NR82
- Molecular Weight:
- 102178.015 Da
References
- Gibor G, Yakubovich D, Peretz A, Attali B: External barium affects the gating of KCNQ1 potassium channels and produces a pore block via two discrete sites. J Gen Physiol. 2004 Jul;124(1):83-102. [15226366 ]