| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2013-04-25 07:28:09 UTC |
|---|
| Update Date | 2014-12-24 20:26:32 UTC |
|---|
| Accession Number | T3D3943 |
|---|
| Identification |
|---|
| Common Name | 2,5-Pyridinedicarboxylic acid, dipropyl ester |
|---|
| Class | Small Molecule |
|---|
| Description | 2,5-Pyridinedicarboxylic acid, dipropyl ester is also known as Repellent MGK 326. It was initially registered by the USDA in 1957 as an insect repellent for livestock. MGK Repellent 326 works to broaden the spectrum of repellency of insect repellents, such as DEET (N,N-diethyl-m-toluamide) or pyrethrins, to repel flies, gnats, and other flying and biting insects. It functions as an insect attractant, an insect repellent and chemosterilant. It is still widely used as an insect repellent. |
|---|
| Compound Type | - Ester
- Ether
- Household Toxin
- Lachrymator
- Organic Compound
- Synthetic Compound
|
|---|
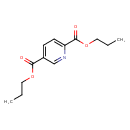
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2,5-PCADPE | | 2,5-Pyridinedicarboxylate, dipropyl ester | | 36169_RIEDEL | | Di-n-propyl 2,5-pyridinedicarboxylate | | Di-n-propyl 2,5-pyridinedicarboxylic acid | | di-n-propyl isocinchomeronate | | Di-n-propyl isocinchomeronate | | Di-propylisocinchomeronate | | Di-propylisocinchomeronic acid | | Diisopropyl pyridine-2,5-dicarboxylate | | Diisopropyl pyridine-2,5-dicarboxylic acid | | Dipropyl 2,5-pyridinedicarboxylate | | Dipropylisocinchomeronate | | Dipropylisocinchomeronic acid | | MGK 326 | | MGK Repellent 326 |
|
|---|
| Chemical Formula | C13H17NO4 |
|---|
| Average Molecular Mass | 251.278 g/mol |
|---|
| Monoisotopic Mass | 251.116 g/mol |
|---|
| CAS Registry Number | 136-45-8 |
|---|
| IUPAC Name | 2,5-dipropyl pyridine-2,5-dicarboxylate |
|---|
| Traditional Name | 2,5-dipropyl pyridine-2,5-dicarboxylate |
|---|
| SMILES | CCCOC(=O)C1=CC=C(N=C1)C(=O)OCCC |
|---|
| InChI Identifier | InChI=1S/C13H17NO4/c1-3-7-17-12(15)10-5-6-11(14-9-10)13(16)18-8-4-2/h5-6,9H,3-4,7-8H2,1-2H3 |
|---|
| InChI Key | InChIKey=IITCWRFYJWUUPC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyridinecarboxylic acids. Pyridinecarboxylic acids are compounds containing a pyridine ring bearing a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyridinecarboxylic acids and derivatives |
|---|
| Direct Parent | Pyridinecarboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridine carboxylic acid
- Dicarboxylic acid or derivatives
- Heteroaromatic compound
- Carboxylic acid ester
- Azacycle
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | 278 °C | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udl-3590000000-335283d5c3f5b46e5b58 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-8920000000-9f595dfb716e05fd030a | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9400000000-3c3114ef191182e79d27 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0f6x-1890000000-a12c2b09a85d2b9d46c3 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-1950000000-5fc268f83e9ea6969beb | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00xr-3900000000-af8fe618b77fd3075e61 | 2016-08-03 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 90 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Dermal |
|---|
| Mechanism of Toxicity | Not known. |
|---|
| Metabolism | Rapidly hydrolyzed to 2,5-pyridinedicarboxylic acid.
|
|---|
| Toxicity Values | LD50: 5230 mg/kg (Oral, Rat)
LD50 9500 mg/kg (Dermal, Rat)
|
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not listed by IARC. The EPA classifies it as a Tentative Class B Carcinogen. |
|---|
| Uses/Sources | Insect repellent, found in many commercial insect repellents and mosquito sprays.
|
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | May cause respiratory tract irritation and skin irritation. May also cause eye irritation. Harmful if swallowed. Rats tolerated MGK-326 in the diet for two years up to 100 mg/kg/day. MGK-326 increased the incidence of liver tumors (males and females) in the 100 mg/kg/day group. The tumor rates in both sexes in the 65 and 250 mg/kg/day groups were comparable to those of the control group. The insect repellent produced increases in the incidence of benign interstitial cell tumors in the testis and benign uterine tumors. Group mean body weights were significantly decreased for males and females at the 1000 mg/kg/day dosage level when compared to the controls. There was no observed teratogenicity. |
|---|
| Symptoms | Not Available |
|---|
| Treatment | EYES: Hold eye open and rinse slowly and gently with water for 15-20 minutes. Remove contact lenses, if present, after the first 5 minutes, then continue rinsing eye. Call a poison control center or doctor for treatment advice.
SKIN: Take off contaminated clothing. Rinse skin immediately with plenty of water for 15-20 minutes. Call a poison control center or doctor for treatment advice.
INHALATION: Move person to fresh air. If person is not breathing, call 911 or an ambulance, then give artificial respiration, preferably by mouth-to-mouth if possible. Call a poison control center or doctor for further treatment advice.
INGESTION: Call a poison control center or doctor immediately for treatment advice. Have person sip a glass of water if able to swallow. Do not induce vomiting unless told to do so by a physicican or poison control centre. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 8693 |
|---|
| ChEMBL ID | CHEMBL1899338 |
|---|
| ChemSpider ID | 8369 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3943.pdf |
|---|
| General References | - Katz TM, Miller JH, Hebert AA: Insect repellents: historical perspectives and new developments. J Am Acad Dermatol. 2008 May;58(5):865-71. doi: 10.1016/j.jaad.2007.10.005. Epub 2008 Feb 13. [18272250 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|