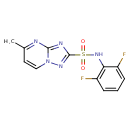| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2013-04-25 07:56:51 UTC |
|---|
| Update Date | 2014-12-24 20:26:33 UTC |
|---|
| Accession Number | T3D3845 |
|---|
| Identification |
|---|
| Common Name | Flumetsulam |
|---|
| Class | Small Molecule |
|---|
| Description | Flumetsulam is a sulfonanilide herbicide that is used for post-emergence control for undersown wheat and certain legume crops and pastures. |
|---|
| Compound Type | - Amide
- Herbicide
- Organic Compound
- Organofluoride
- Pesticide
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Broadstrike | | N-(2,6-Difluorophenyl)-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidine-2-sulfonamide | | N-(2,6-Difluorophenyl)-5-methyl[1,2,4]triazolo-[1,5-a]pyrimidine-2-sulfonamide | | N-(2,6-Difluorophenyl)-5-methyl[1,2,4]triazolo[1,5-a]pyrimidine-2-sulfonamide |
|
|---|
| Chemical Formula | C12H9F2N5O2S |
|---|
| Average Molecular Mass | 325.294 g/mol |
|---|
| Monoisotopic Mass | 325.045 g/mol |
|---|
| CAS Registry Number | 98967-40-9 |
|---|
| IUPAC Name | N-(2,6-difluorophenyl)-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidine-2-sulfonamide |
|---|
| Traditional Name | N-(2,6-difluorophenyl)-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidine-2-sulfonamide |
|---|
| SMILES | CC1=NC2=NC(=NN2C=C1)S(=O)(=O)NC1=C(F)C=CC=C1F |
|---|
| InChI Identifier | InChI=1S/C12H9F2N5O2S/c1-7-5-6-19-11(15-7)16-12(17-19)22(20,21)18-10-8(13)3-2-4-9(10)14/h2-6,18H,1H3 |
|---|
| InChI Key | InChIKey=RXCPQSJAVKGONC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2,4-triazolopyrimidine-2-sulfonanilides. These are polycyclic aromatic compounds containing a 1,2,4-triazolo[1,5-a]pyrimidine ring system, which is substituted with a sulfonanilide at the 2-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Triazolopyrimidines |
|---|
| Sub Class | 1,2,4-triazolopyrimidine-2-sulfonamides |
|---|
| Direct Parent | 1,2,4-triazolopyrimidine-2-sulfonanilides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2,4-triazolopyrimidine-2-sulfonanilide
- Sulfanilide
- Halobenzene
- Fluorobenzene
- Pyrimidine
- Aryl fluoride
- Organosulfonic acid amide
- Benzenoid
- Aryl halide
- Monocyclic benzene moiety
- 1,2,4-triazole
- Triazole
- Aminosulfonyl compound
- Organosulfonic acid or derivatives
- Organic sulfonic acid or derivatives
- Heteroaromatic compound
- Azole
- Sulfonyl
- Azacycle
- Organonitrogen compound
- Organofluoride
- Organohalogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organosulfur compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0109000000-1acfb433a6cbb356b9fa | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004l-0902000000-2b2befb6c154013b9ff7 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-5900000000-f942b5059536cd8fcc93 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0309000000-68074fb6f654cca3a8e5 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-7d3062aa06fd3a1cbac9 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0900000000-e81067d1fe60c7bbd557 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 91759 |
|---|
| ChEMBL ID | CHEMBL1389671 |
|---|
| ChemSpider ID | 82857 |
|---|
| KEGG ID | C18852 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3845.pdf |
|---|
| General References | Not Available |
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|