| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2010-05-04 17:21:10 UTC |
|---|
| Update Date | 2014-12-24 20:26:27 UTC |
|---|
| Accession Number | T3D3728 |
|---|
| Identification |
|---|
| Common Name | Alternariol methyl ether |
|---|
| Class | Small Molecule |
|---|
| Description | Alternariol methyl ether is an altertoxin, which is a mycotoxin of Alternaria fungi. Altertoxins are important contaminants in cereals, vegetables, and fruits, as well as in the ground, on wood or walls. Studies have shown altertoxins to be toxic, genotoxic, mutagenic, and carcinogenic. In particular, they have been associated with esophageal cancer in humans. (1, 3) |
|---|
| Compound Type | - Ester
- Ether
- Fungal Toxin
- Mycotoxin
- Natural Compound
- Organic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2-Biphenylcarboxylic acid, 2',3',4'-trihydroxy-5-methoxy-6'-methyl-, delta-lactone | | 2-Biphenylcarboxylic acid, 2',3',4'-trihydroxy-5-methoxy-6'-methyl-, delta-lactone (6CI) | | 3,7-Dihydroxy-9-methoxy-1-methyl-6H-benzo[c]chromen-6-one | | 3,7-Dihydroxy-9-methoxy-1-methyl-6H-dibenzo(b,d)pyran-6-one | | alternariol 5-O-methyl ether | | Alternariol monomethyl ether | | Alternariol monomethylether | | Alternariol-9-methyl ether | | AME | | Djalonensone |
|
|---|
| Chemical Formula | C15H12O5 |
|---|
| Average Molecular Mass | 272.253 g/mol |
|---|
| Monoisotopic Mass | 272.068 g/mol |
|---|
| CAS Registry Number | 23452-05-3 |
|---|
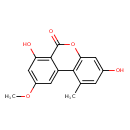
| IUPAC Name | 3,7-dihydroxy-9-methoxy-1-methyl-6H-benzo[c]chromen-6-one |
|---|
| Traditional Name | 3,7-dihydroxy-9-methoxy-1-methylbenzo[c]chromen-6-one |
|---|
| SMILES | COC1=CC(O)=C2C(=O)OC3=C(C(C)=CC(O)=C3)C2=C1 |
|---|
| InChI Identifier | InChI=1S/C15H12O5/c1-7-3-8(16)4-12-13(7)10-5-9(19-2)6-11(17)14(10)15(18)20-12/h3-6,16-17H,1-2H3 |
|---|
| InChI Key | InChIKey=LCSDQFNUYFTXMT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as coumarins and derivatives. These are polycyclic aromatic compounds containing a 1-benzopyran moiety with a ketone group at the C2 carbon atom (1-benzopyran-2-one). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Coumarins and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Coumarins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coumarin
- Isocoumarin
- Benzopyran
- 2-benzopyran
- 1-benzopyran
- Anisole
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Pyranone
- Benzenoid
- Pyran
- Heteroaromatic compound
- Vinylogous acid
- Lactone
- Oxacycle
- Ether
- Organoheterocyclic compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-9dff801a2758792e7269 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0090000000-607a288b5f5a34f0a6c9 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6u-1090000000-f0add938f0e76c07dcce | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-7173b22425d15c235102 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0090000000-68d751616f54427bceab | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006y-2290000000-c7c7120ed9225f2b0ec5 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral, dermal, inhalation, and parenteral (contaminated drugs). (5) |
|---|
| Mechanism of Toxicity | Alternariol methyl ether has been shown to have genotoxic and mutagenic properties. It has demonstrated DNA-damaging activities such as single-strand and double-strand DNA breaks, DNA-intercalating, and DNA cross-linking, as well as induction of DNA repair synthesis and inhibition of DNA replication. These effects are thought to be at least partially due to its ability to bind to the DNA minor groove with high affinity, which inhibits the activity of DNA-acting enzymes such as topoisomerase. (1, 2, 3) |
|---|
| Metabolism | Alternariol methyl ether is metabolized by microsomes in the liver, preferentially at aromatic positions. It is also demethylated to alternariol. The products of aromatic hydroxylation are either catechols or hydroquinones, which may form reactive semiquinones and quinones or undergo redox cycling. (4) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not listed by IARC. |
|---|
| Uses/Sources | Alternariol methyl ether is an altertoxin, which is a mycotoxin of Alternaria fungi. Altertoxins are important contaminants in cereals, vegetables, and fruits, as well as in the ground, on wood or walls. (1, 3) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Altertoxins have been shown to be toxic, genotoxic, mutagenic, and carcinogenic. In particular, they have been associated with esophageal cancer in humans. (1, 3) |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 5360741 |
|---|
| ChEMBL ID | CHEMBL483526 |
|---|
| ChemSpider ID | 4514573 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3728.pdf |
|---|
| General References | - Fehr M, Pahlke G, Fritz J, Christensen MO, Boege F, Altemoller M, Podlech J, Marko D: Alternariol acts as a topoisomerase poison, preferentially affecting the IIalpha isoform. Mol Nutr Food Res. 2009 Apr;53(4):441-51. doi: 10.1002/mnfr.200700379. [18727009 ]
- Brugger EM, Wagner J, Schumacher DM, Koch K, Podlech J, Metzler M, Lehmann L: Mutagenicity of the mycotoxin alternariol in cultured mammalian cells. Toxicol Lett. 2006 Jul 14;164(3):221-30. Epub 2006 Feb 7. [16464542 ]
- Lehmann L, Wagner J, Metzler M: Estrogenic and clastogenic potential of the mycotoxin alternariol in cultured mammalian cells. Food Chem Toxicol. 2006 Mar;44(3):398-408. Epub 2005 Sep 27. [16194592 ]
- Pfeiffer E, Schebb NH, Podlech J, Metzler M: Novel oxidative in vitro metabolites of the mycotoxins alternariol and alternariol methyl ether. Mol Nutr Food Res. 2007 Mar;51(3):307-16. [17340575 ]
- Peraica M, Domijan AM: Contamination of food with mycotoxins and human health. Arh Hig Rada Toksikol. 2001 Mar;52(1):23-35. [11370295 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|