Anisatin (T3D3074)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-23 18:26:04 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:56 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D3074 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Anisatin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Anisatin is a plant toxin found in the Japanese star anise (Illicium anisatum). The Japanese star anise has been burned as incense in Japan, where it is known as shikimi, as well as used in topical folk remedies. (2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
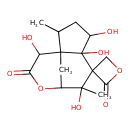
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C15H20O8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 328.315 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 328.116 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 5230-87-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 4',5',7',11'-tetrahydroxy-2',7'-dimethyl-9'-oxaspiro[oxetane-3,6'-tricyclo[6.3.1.0¹,⁵]dodecane]-4,10'-dione | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | 4',5',7',11'-tetrahydroxy-2',7'-dimethyl-9'-oxaspiro[oxetane-3,6'-tricyclo[6.3.1.0¹,⁵]dodecane]-4,10'-dione | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | CC1CC(O)C2(O)C3(COC3=O)C(C)(O)C3CC12C(O)C(=O)O3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C15H20O8/c1-6-3-7(16)15(21)13(6)4-8(23-10(18)9(13)17)12(2,20)14(15)5-22-11(14)19/h6-9,16-17,20-21H,3-5H2,1-2H3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=GEVWHIDSUOMVRI-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as terpene lactones. These are prenol lipids containing a lactone ring. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Prenol lipids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Terpene lactones | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Terpene lactones | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Oral (ingestion) (3) ; dermal (3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Anisatin acts as a GABA system antagonist. It binds non-competitively to the picrotoxinin site of the GABA receptor, prolonging the closed time of the receptor channel and reducing the probability of it opening. (1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Anisatin is a plant toxin found in the Japanese star anise (Illicium anisatum). The Japanese star anise has been burned as incense in Japan, where it is known as shikimi, as well as used in topical folk remedies. (2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Death may result from respiratory paralysis. (2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Symptoms of anisatin poisoning include diarrhea, vomiting, and stomach pain, followed by nervous system excitation, seizures, loss of consciousness. (2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 115121 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C09294 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Anisatin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Anisatin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRA1
- Uniprot ID:
- P14867
- Molecular Weight:
- 51801.395 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA2
- Uniprot ID:
- P47869
- Molecular Weight:
- 51325.85 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA3
- Uniprot ID:
- P34903
- Molecular Weight:
- 55164.055 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA4
- Uniprot ID:
- P48169
- Molecular Weight:
- 61622.645 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Transporter activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA5
- Uniprot ID:
- P31644
- Molecular Weight:
- 52145.645 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRA6
- Uniprot ID:
- Q16445
- Molecular Weight:
- 51023.69 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel (By similarity).
- Gene Name:
- GABRB1
- Uniprot ID:
- P18505
- Molecular Weight:
- 54234.085 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB2
- Uniprot ID:
- P47870
- Molecular Weight:
- 59149.895 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Gaba-gated chloride ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRB3
- Uniprot ID:
- P28472
- Molecular Weight:
- 54115.04 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRD
- Uniprot ID:
- O14764
- Molecular Weight:
- 50707.835 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRE
- Uniprot ID:
- P78334
- Molecular Weight:
- 57971.175 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG1
- Uniprot ID:
- Q8N1C3
- Molecular Weight:
- 53594.49 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- Component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the vertebrate brain. Functions also as histamine receptor and mediates cellular responses to histamine. Functions as receptor for diazepines and various anesthetics, such as pentobarbital; these are bound at a separate allosteric effector binding site. Functions as ligand-gated chloride channel.
- Gene Name:
- GABRG2
- Uniprot ID:
- P18507
- Molecular Weight:
- 54161.78 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Inhibitory extracellular ligand-gated ion channel activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRG3
- Uniprot ID:
- Q99928
- Molecular Weight:
- 54288.16 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. In the uterus, the function of the receptor appears to be related to tissue contractility. The binding of this pI subunit with other GABA(A) receptor subunits alters the sensitivity of recombinant receptors to modulatory agents such as pregnanolone.
- Gene Name:
- GABRP
- Uniprot ID:
- O00591
- Molecular Weight:
- 50639.735 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-1 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR1
- Uniprot ID:
- P24046
- Molecular Weight:
- 55882.91 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel. Rho-2 GABA receptor could play a role in retinal neurotransmission.
- Gene Name:
- GABRR2
- Uniprot ID:
- P28476
- Molecular Weight:
- 54150.41 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Gaba-a receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRR3
- Uniprot ID:
- A8MPY1
- Molecular Weight:
- 54271.1 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]
- General Function:
- Transmembrane signaling receptor activity
- Specific Function:
- GABA, the major inhibitory neurotransmitter in the vertebrate brain, mediates neuronal inhibition by binding to the GABA/benzodiazepine receptor and opening an integral chloride channel.
- Gene Name:
- GABRQ
- Uniprot ID:
- Q9UN88
- Molecular Weight:
- 72020.875 Da
References
- Ikeda T, Ozoe Y, Okuyama E, Nagata K, Honda H, Shono T, Narahashi T: Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons. Br J Pharmacol. 1999 Aug;127(7):1567-76. [10455311 ]