| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-19 21:58:54 UTC |
|---|
| Update Date | 2014-12-24 20:24:06 UTC |
|---|
| Accession Number | T3D1522 |
|---|
| Identification |
|---|
| Common Name | Aluminium sulfate |
|---|
| Class | Small Molecule |
|---|
| Description | Aluminium sulfate is used in foods as a firming agent. Aluminium sulfate, written as Al2(SO4)3 or Al2O12S3 Aluminium sulfate is an industrial chemical used as a flocculating agent in the purification of drinking water and waste water treatment plants, and also in paper manufacturing. In construction industry it is used as waterproofing agent and accelerator in concrete. Another use is a foaming agent in fire fighting foam.
Aluminium sulfate belongs to the family of Post-transition Metal Sulfates. These are inorganic compounds in which the largest oxoanion is sulfate, and in which the heaviest atom not in an oxoanion is a post-transitional metal. |
|---|
| Compound Type | - Aluminum Compound
- Food Toxin
- Household Toxin
- Industrial/Workplace Toxin
- Inorganic Compound
- Metabolite
- Natural Compound
|
|---|
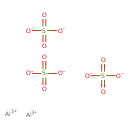
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Aluminium sulfic acid | | Aluminium sulphate | | Aluminium sulphic acid | | Aluminum sulfate | | Aluminum sulfate (3:2) | | Aluminum sulphate | | Aluminum sulphate (3:2) | | Aluminum trisulfate | | Aluminum trisulphate | | Alunogenite | | Dialuminum sulfate | | Dialuminum sulphate | | Dialuminum trisulfate | | Dialuminum trisulphate | | E520 | | Sulfuric acid aluminum(3+) salt (3:2) | | Sulfuric acid, aluminum potassium salt (2:1:1) | | Sulfuric acid, aluminum salt (3:2) | | Sulfuric acid, aluminum(3+) salt (3:2) |
|
|---|
| Chemical Formula | Al2O12S3 |
|---|
| Average Molecular Mass | 342.151 g/mol |
|---|
| Monoisotopic Mass | 341.818 g/mol |
|---|
| CAS Registry Number | 10043-01-3 |
|---|
| IUPAC Name | dialuminium(3+) ion trisulfate |
|---|
| Traditional Name | dialuminium(3+) ion trisulfate |
|---|
| SMILES | [Al+3].[Al+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O |
|---|
| InChI Identifier | InChI=1S/2Al.3H2O4S/c;;3*1-5(2,3)4/h;;3*(H2,1,2,3,4)/q2*+3;;;/p-6 |
|---|
| InChI Key | InChIKey=DIZPMCHEQGEION-UHFFFAOYSA-H |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as post-transition metal sulfates. These are inorganic compounds in which the largest oxoanion is sulfate, and in which the heaviest atom not in an oxoanion is a post-transition metal. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Mixed metal/non-metal compounds |
|---|
| Class | Post-transition metal oxoanionic compounds |
|---|
| Sub Class | Post-transition metal sulfates |
|---|
| Direct Parent | Post-transition metal sulfates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Post-transition metal sulfate
- Inorganic post-transition metal salt
- Inorganic oxide
- Inorganic salt
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cell surface
- Cytoplasm
- Cytosol
- Endoplasmic reticulum
- Extracellular
- Extracellular matrix
- Golgi apparatus
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Apoptosis | Not Available | map04210 |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White crystals. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 770°C (decomp, anhydrous); 86.5°C (octadecahydrate) | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-ac4e3d4b02133ae3ad7c | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0009000000-ac4e3d4b02133ae3ad7c | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0009000000-ac4e3d4b02133ae3ad7c | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-bdd76efeae52ab20e3a9 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0009000000-bdd76efeae52ab20e3a9 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0009000000-bdd76efeae52ab20e3a9 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (8) ; inhalation (8) |
|---|
| Mechanism of Toxicity | The main target organs of aluminum are the central nervous system and bone. Aluminum binds with dietary phosphorus and impairs gastrointestinal absorption of phosphorus. The decreased phosphate body burden results in osteomalacia (softening of the bones due to defective bone mineralization) and rickets. Aluminum's neurotoxicity is believed to involve several mechanisms. Changes in cytoskeletal protein functions as a results of altered phosphorylation, proteolysis, transport, and synthesis are believed to be one cause. Aluminum may induce neurobehavioral effects by affecting permeability of the blood-brain barrier, cholinergic activity, signal transduction pathways, lipid peroxidation, and impair neuronal glutamate nitric oxide-cyclic GMP pathway, as well as interfere with metabolism of essential trace elements because of similar coordination chemistries and consequent competitive interactions. It has been suggested that aluminum's interaction with estrogen receptors increases the expression of estrogen-related genes and thereby contributes to the progression of breast cancer (1), but studies have not been able to establish a clear link between aluminum and increased risk of breast cancer (4). Certain aluminum salts induce immune responses by activating inflammasomes. (8, 1, 2) |
|---|
| Metabolism | Aluminum is poorly absorbed following either oral or inhalation exposure and is essentially not absorbed dermally. The bioavailability of aluminum is strongly influenced by the aluminum compound and the presence of dietary constituents which can complex with aluminum and enhance or inhibit its absorption. Aluminum binds to various ligands in the blood and distributes to every organ, with highest concentrations found in bone and lung tissues. In living organisms, aluminum is believed to exist in four different forms: as free ions, as low-molecular-weight complexes, as physically bound macromolecular complexes, and as covalently bound macromolecular complexes. Absorbed aluminum is excreted principally in the urine and, to a lesser extent, in the bile, while unabsorbed aluminum is excreted in the faeces. (8) |
|---|
| Toxicity Values | LD50: 980 mg/kg (Oral, Mouse) (2)
LD50: 40 mg/kg (Intraperitoneal, Mouse) (3) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not listed by IARC. IARC classified aluminum production as carcinogenic to humans (Group 1), but did not implicate aluminum itself as a human carcinogen. (11) A link between use of aluminum-containing antiperspirants and increased risk of breast cancer has been proposed (1), but studies have not been able to establish a clear link (4). |
|---|
| Uses/Sources | Aluminium sulfate is an industrial chemical used as a flocculating agent in the purification of drinking water, in waste water treatment plants, and also in paper manufacturing. (10) |
|---|
| Minimum Risk Level | Intermediate Oral: 1.0 mg/kg/day (7)
Chronic Oral: 1.0 mg/kg/day (7) |
|---|
| Health Effects | Aluminum targets the nervous system and causes decreased nervous system performance and is associated with altered function of the blood-brain barrier. The accumulation of aluminum in the body may cause bone or brain diseases. High levels of aluminum have been linked to Alzheimer's disease. A small percentage of people are allergic to aluminium and experience contact dermatitis, digestive disorders, vomiting or other symptoms upon contact or ingestion of products containing aluminium. (8, 9) |
|---|
| Symptoms | Inhalating aluminum dust causes coughing and abnormal chest X-rays. A small percentage of people are allergic to aluminium and experience contact dermatitis, digestive disorders, vomiting or other symptoms upon contact or ingestion of products containing aluminium. (8, 9) |
|---|
| Treatment | EYES: irrigate opened eyes for several minutes under running water. INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice. SKIN: should be treated immediately by rinsing the affected parts in cold running water for at least 15 minutes, followed by thorough washing with soap and water. If necessary, the person should shower and change contaminated clothing and shoes, and then must seek medical attention. INHALATION: supply fresh air. If required provide artificial respiration. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB34734 |
|---|
| PubChem Compound ID | 24850 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 23233 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 74768 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | C041524 |
|---|
| Stitch ID | Aluminium sulfate |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 12372 |
|---|
| Wikipedia Link | Aluminium_sulfate |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D1522.pdf |
|---|
| General References | - Darbre PD: Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J Appl Toxicol. 2006 May-Jun;26(3):191-7. [16489580 ]
- Aimanianda V, Haensler J, Lacroix-Desmazes S, Kaveri SV, Bayry J: Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol Sci. 2009 Jun;30(6):287-95. doi: 10.1016/j.tips.2009.03.005. Epub 2009 May 11. [19439372 ]
- Wu Q, Blakeley LR, Cornwall MC, Crouch RK, Wiggert BN, Koutalos Y: Interphotoreceptor retinoid-binding protein is the physiologically relevant carrier that removes retinol from rod photoreceptor outer segments. Biochemistry. 2007 Jul 24;46(29):8669-79. Epub 2007 Jun 30. [17602665 ]
- Willhite CC, Karyakina NA, Yokel RA, Yenugadhati N, Wisniewski TM, Arnold IM, Momoli F, Krewski D: Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit Rev Toxicol. 2014 Oct;44 Suppl 4:1-80. doi: 10.3109/10408444.2014.934439. [25233067 ]
- WHO (1997). Environmental Health Criteria 194: Aluminum.
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for aluminum. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Aluminium. Last Updated 16 June 2009. [Link]
- Wikipedia. Aluminium sulfate. Last Updated 14 June 2009. [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|