| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-02 22:33:20 UTC |
|---|
| Update Date | 2014-12-24 20:22:51 UTC |
|---|
| Accession Number | T3D0806 |
|---|
| Identification |
|---|
| Common Name | Methylchlorophenoxypropionic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Mecoprop, or methylchlorophenoxypropionic acid (MCPP), is a common general use herbicide found in many household weed killers and weed-and-feed type lawn fertilizers. It is primarily used to control broadleaf weeds. It is often used in combination with other chemically related herbicides such as 2,4-D, dicamba, and MCPA.The United States Environmental Protection Agency has classified mecoprop as toxicity class III - slightly toxic. Mecoprop is a mixture of two stereoisomers, with the (R)-(+)-enantiomer ('Mecoprop-P', 'Duplosan KV') possessing the herbicidal activity |
|---|
| Compound Type | - Aromatic Hydrocarbon
- Ether
- Household Toxin
- Industrial/Workplace Toxin
- Organic Compound
- Organochloride
- Pesticide
- Synthetic Compound
|
|---|
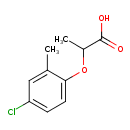
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (+ )-a-(4-chloro-2-Methylphenoxy) propionic acid | | (+)-.alpha.-(4-chloro-2-methylphenoxy) propionic acid | | (+)-a-(4-chloro-2-Methylphenoxy) propionic acid | | (+)-alpha-(4-chloro-2-Methylphenoxy) propionic acid | | (+-)-2-((4-chloro-O-Tolyl)oxy)propionic acid | | (4-chloro-2-Methylphenoxy)propionic acid | | 2-(2'-Methyl-4'-chlorophenoxy)propionic acid | | 2-(2-Methyl-4-chlorophenoxy)propionic acid | | 2-(4-Chloor-2-methyl-fenoxy)-propionzuur | | 2-(4-Chlor-2-methyl-phenoxy)-propionsaeure | | 2-(4-chloro-2-Methylphenoxy)propanoic acid | | 2-(4-chloro-2-Methylphenoxy)propionic acid | | 2-(4-chloro-2-Tolyloxy)propionic acid | | 2-(4-chloro-O-Tolyl)oxylpropionic acid | | 2-(4-chloro-O-Tolyloxy)propionic acid | | 2-(4-Chlorophenoxy-2-methyl)propionic acid | | 2-(P-chloro-O-Tolyloxy)propionic acid | | 2-MCPP | | 2-Methyl-4-chlorophenoxy-.alpha.-propionic acid | | 2-Methyl-4-chlorophenoxy-a-propionic acid | | 4-chloro-2-Methylphenoxy-.alpha.-propionic acid | | 4-chloro-2-Methylphenoxy-a-propionic acid | | 4-chloro-2-Methylphenoxy-alpha-propionic acid | | a-(2-Methyl-4-chlorophenoxy)propionic acid | | a-(4-chloro-2-Methylphenoxy)propionic acid | | alpha-(2-Methyl-4-chlorophenoxy)propionic acid | | Alpha.-(2-methyl-4-chlorophenoxy)propionic acid | | Anicon b | | Anicon p | | Astix | | BH mecoprop | | Caswell No. 559 | | Celatox CMPP | | Chipco turf herbicide MCPP | | CMPP | | Compitox | | Duplosan CMPP | | Duplosan new system CMPP | | FBC CMPP | | Hedonal | | Hedonal MCPP | | Iso-cornox | | Isocarnox | | Kilprop | | Liranox | | MCPP | | MCPP 2,4-D | | MCPP-D-4 | | MCPP-K-4 | | Mechlorprop | | Mecomec | | Mecopar | | Mecopeop | | Mecoper | | Mecopex | | Mecoprop | | Mecoprop-p | | Mecoturf | | Mecprop | | Mepro | | Methoxone | | Methylchlorophenoxypropionate | | Morogal | | Mwcoprop | | N.b. mecoprop | | O-(4-chloro-2-Methylphenyl)lactic acid | | Propionic acid, 2-(4-chloro-2-methylphenoxy) | | Propionic acid, {2-[(4-chloro-O-tolyl)oxy]-} | | Proponex-plus | | Rankotex | | Runcatex |
|
|---|
| Chemical Formula | C10H11ClO3 |
|---|
| Average Molecular Mass | 214.646 g/mol |
|---|
| Monoisotopic Mass | 214.040 g/mol |
|---|
| CAS Registry Number | 93-65-2 |
|---|
| IUPAC Name | 2-(4-chloro-2-methylphenoxy)propanoic acid |
|---|
| Traditional Name | mecoprop |
|---|
| SMILES | CC(OC1=CC=C(Cl)C=C1C)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H11ClO3/c1-6-5-8(11)3-4-9(6)14-7(2)10(12)13/h3-5,7H,1-2H3,(H,12,13) |
|---|
| InChI Key | InChIKey=WNTGYJSOUMFZEP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2-phenoxypropionic acids. These are aromatic compounds hat contain a phenol ether attached to the C2-atom of a phenylpropionic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | 2-phenoxypropionic acids |
|---|
| Direct Parent | 2-phenoxypropionic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-phenoxypropionic acid
- Phenoxyacetate
- Phenoxy compound
- Phenol ether
- Alkyl aryl ether
- Toluene
- Chlorobenzene
- Halobenzene
- Aryl chloride
- Aryl halide
- Carboxylic acid derivative
- Carboxylic acid
- Ether
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Organic oxide
- Organochloride
- Hydrocarbon derivative
- Organohalogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 94.5°C | | Boiling Point | Not Available | | Solubility | 0.62 mg/mL at 20°C [MARTIN,H & WORTHING,CR (1977)] | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-1900000000-ac5895b5fc793274272e | 2021-09-23 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-04 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-04 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-0006-0900000000-11480ccdc682acf643a4 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-0006-0900000000-35ac6c6fa42b085bff76 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-0006-0900000000-a5fe008b371ac3a09068 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-01ox-0940000000-d67151b96f4db9b5c8fe | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-0006-0900000000-7c2c26ebad0bbc389d73 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-0006-0900000000-78fe325c97eeb615bb6d | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-0006-0900000000-d1d892cf06b30e189f20 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-0006-0900000000-f13d6ff14498342f7f7f | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-0006-0900000000-cc43b19d54523b6fdb91 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-01ox-0940000000-1991429b9e6b1d34daf3 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-0006-0900000000-bb036567f924f1bfd16f | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-0006-0900000000-3ea2459793789ac2ab76 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-0006-0900000000-4075a19636afe9c55f70 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Negative | splash10-0006-0900000000-ff229e048896cb82eea8 | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-1980000000-8c7b5fb29aab1f034b32 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kf-2910000000-832e0bf62c4c2e533d5d | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ox-3900000000-5181e68db5f5e77a3c05 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0290000000-8bd0d82fa7cbbb521f18 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ox-0930000000-d64f57906ccd7632a322 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-1900000000-32d683cd7014a51a8388 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kf-0920000000-3ebee7efd38e453bedba | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kf-0900000000-11415a668ebe7ae530dd | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9800000000-49b96972eeb200dc85ef | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-2920000000-143d9ac5b3dca1a57235 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9000000000-64d108b3e48a8092bcb9 | 2021-10-12 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0006-4910000000-7bdfa1d9086c4836e053 | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | CDDs cause their toxic effects by binding to the aryl hydrocarbon receptor and subsequently altering the trascription of certain genes. The affinity for the Ah receptor depends on the structure of the specific CDD. The change in gene expression may result from the direct interaction of the Ah receptor and its heterodimer-forming partner, the aryl hydrocarbon receptor nuclear translocator, with gene regulatory elements or the initiation of a phosphorylation/dephosphorylation cascade that subsequently activates other transcription factors. The affected genes include several oncogenes, growth factors, receptors, hormones, and drug-metabolizing enzymes. The change in transcription/translation of these genes is believed to be the cause of most of the toxic effects of CDDs. This includes 2,3,7,8-tetrachlorodibenzo-p-dioxin's carcinogenicity is thought to be the result of its ability to alter the capacity of both exogenous and endogenous substances to damage the DNA by inducing CYP1A1- and CYP1A2-dependent drug-metabolizing enzymes. (1) |
|---|
| Metabolism | CDDs are absorbed through oral, inhalation, and dermal routes of exposure. CDDs are carried in the plasma by serum lipids and lipoproteins, distributing mainly to the liver and adipose tissue. CDDs are very slowly metabolized by the microsomal monooxygenase system to polar metabolites that can undergo conjugation with glucuronic acid and glutathione. They may increase the rate of their own metabolism by inducing CDDs induce both phase I and phase II enzymes. The major routes of excretion of CDDs are the bile and the feces, though smaller amounts are excreted in the urine and via lactation. (1) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (4) |
|---|
| Uses/Sources | Dioxins occur as by-products from the manufacture of organochlorides, the bleaching of paper, chlorination by waste and drinking water treatment plants, municipal solid waste and industrial incinerators, and natural sources such as volcanoes and forest fires. (1, 2) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Exposure to large amounts of CDDs causes chloracne, a severe skin disease with acne-like lesions that occur mainly on the face and upper body. CDDs may also cause liver damage and induce long-term alterations in glucose metabolism and subtle changes in hormonal levels. In addition, studies have shown that CDDs may disrupt the endocrine system and weaken the immune system, as well as cause reproductive damage and birth defects, central and peripheral nervous system pathology, thyroid disorders, endometriosis, and diabetes. 2,3,7,8-Tetrachlorodibenzo-p-dioxin is also a known human carcinogen. (1, 2) |
|---|
| Symptoms | In addition to chloracne, CDD exposure causes skin rashes, discoloration, and excessive body hair. (1) |
|---|
| Treatment | Treatment may include washing any areas of contact, GI decontamination if swallowed, administering an IV and forced alkaline diuresis. (3) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 7153 |
|---|
| ChEMBL ID | CHEMBL272942 |
|---|
| ChemSpider ID | 6886 |
|---|
| KEGG ID | C18742 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 75704 |
|---|
| BioCyc ID | CPD0-1524 |
|---|
| CTD ID | C008340 |
|---|
| Stitch ID | Methylchlorophenoxypropionic acid |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 6483 |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - ATSDR - Agency for Toxic Substances and Disease Registry (1998). Toxicological profile for chlorinated dibenzo-p-dioxins (CDDs). U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Polychlorinated dibenzodioxins. Last Updated 19 May 2009. [Link]
- US Environmental Protection Agency (2009). Recognition and Management of Pesticide Poisonings. [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|